A redox-active organic cation for safer high energy density Li-ion batteries†
Abstract
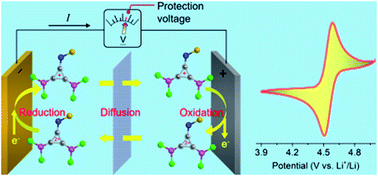
Ni-rich layered cathode materials are at the forefront to be deployed in high energy density Li-ion batteries for the automotive market. However, the intrinsic poor structural and interfacial stability during overcharging could trigger violent thermal failure, which severely limits their wide application. To protect the Ni-rich cathode from overcharging, we firstly report a redox-active cation, thioether-substituted diaminocyclopropenium, as an electrolyte additive to limit the cell voltage within the safe value during overcharging. The organic cation demonstrates a record-breaking electrochemical reversibility at ∼4.55 V versus Li+/Li and solubility (0.5 M) in carbonate-based electrolyte. The protection capability of the additive was explored in two cell chemistries: a LiNi0.8Co0.15Al0.05O2/graphite cell and a LiNi0.8Co0.15Al0.05O2/silicon–graphene cell with areal capacities of ∼2.2 mA h cm−2 and ∼3 mA h cm−2, respectively. With 0.2 M addition, the LiNi0.8Co0.15Al0.05O2/graphite cell survived 54 cycles at 0.2C with 100% overcharge. Moreover, the cell can carry an utmost 4.4 mA cm−2 (2C) with 100% overcharge and a maximum capacity of 7540% SOC at 0.2C.


 Please wait while we load your content...
Please wait while we load your content...