Sacrificial agent-free photocatalytic H2O2 evolution via two-electron oxygen reduction using a ternary α-Fe2O3/CQD@g-C3N4 photocatalyst with broad-spectrum response†
Abstract
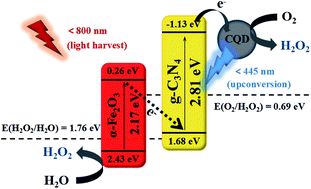
Ultrathin g-C3N4 nanosheets have been fabricated via a two-step calcination regulated by melamine precursors at a high heating rate (30 °C min−1). The resulting g-C3N4 nanosheets were further employed as carriers for the growth of carbon quantum dots (CQDs) and (110) exposed α-Fe2O3 through the PVP-enabled adsorption effects by a solvothermal process. It was discovered that the so fabricated ternary photocatalyst α-Fe2O3/CQD@g-C3N4 presented a broad-spectrum absorption range (up to 800 nm) and particularly enhanced active sites of photogenerated electrons for highly efficient photocatalytic oxygen reduction toward H2O2 evolution in pure water. A H2O2 production rate of 1.16 μM min−1 could be expected for the developed photocatalyst under visible light irradiation, which is about 19 times faster than that of pure ultrathin g-C3N4. Herein, the loaded Fe2O3 could transform the H2O2 evolution from two-step single-electron reduction into one-step two-electron one, as verified by the various active species experiments and rotating ring-disk electrode tests. This work presents a new perspective in designing ultrathin g-C3N4 through a simple method of precursor-regulated calcination, which features more outstanding advantages than the conventional exfoliation of bulk g-C3N4 towards ultrathin g-C3N4. More importantly, it provides an optimized photocatalytic reaction route of two-electron oxygen reduction for efficient H2O2 production in pure water under visible light irradiation, without the need for noble metals or organic sacrificial agents.


 Please wait while we load your content...
Please wait while we load your content...