Oxygen defect chemistry for the reversible transformation of titanates for sizeable potassium storage†
Abstract
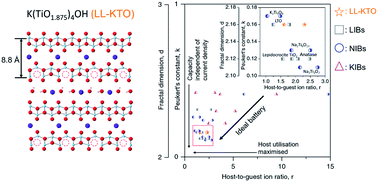
Potassium-ion batteries (KIBs) are promising substitutes for lithium-ion batteries (LIBs) due to the earth-abundancy of potassium. However, practical KIB applications are hindered by slow diffusion kinetics and severe structural deterioration as the large cation is cycled in and out of the electrode. Here, a high-capacity electrode, oxygen-deficient loose-layered potassium titanate (LL-KTO), is synthesized to electrochemically store potassium via a “stacked ↔ sliced structural transformation” with net-zero structural deterioration. Owing to the positive structural energy compensation from oxygen vacancies, LL-KTO delaminates and restacks with K+ ions reversibly upon charging and discharging, in contrast to rigid oxide electrodes. As a result, it achieves a capacity of 201 mA h g−1 over 1800 cycles at 100 mA g−1, on par with values for titanium-oxide based LIBs. Peukert's constant, fractal dimension and host-to-guest ion ratio are further demonstrated as matrices to evaluate the performances of electrodes and show that LL-KTO with stacked ↔ sliced structural transformation pushes the kinetic boundary and approaches the thermodynamic limit of ion batteries. This work addresses the disadvantages of large ion storage by designing a new ion-storing mechanism and provides an important guide to design future energy storage systems and a method to compare electrode materials across different systems.


 Please wait while we load your content...
Please wait while we load your content...