Effects of a solid electrolyte coating on the discharge kinetics of a LiCoO2 electrode: mechanism and potential applications†
Abstract
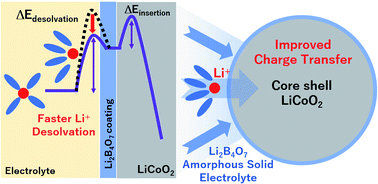
The application of a Li+-conductive amorphous Li2B4O7 coating on a LiCoO2 electrode enhanced its discharge kinetics by increasing the local concentration of Li+ at the surface of LiCoO2 particles. The origin of internal resistance in Li+ intercalation steps was elucidated by electrochemical impedance spectroscopy (EIS)-based characterization of discharge kinetics for states of charges of 0, 50, and 100%, while the activation energies of intercalation steps were determined from EIS data collected at different temperatures (−10, 0, 20, and 40 °C). The activation energy of Li+ desolvation was smaller than that previously reported for bare LiCoO2 particles, which suggested that the significant changes in kinetics associated with polarization mitigation were due to the Li+ exchange reaction (Li+ adsorption and diffusion processes) on the surface of LiCoO2 particles. Finally, C-rate capability tests performed at −10 °C revealed that the capacity retention of the electrode comprising Li2B4O7-coated LiCoO2 particles exceeded that of the electrode comprising bare LiCoO2 particles (45% vs. 18%, respectively).


 Please wait while we load your content...
Please wait while we load your content...