Quantifying the impact of disorder on Li-ion and Na-ion transport in perovskite titanate solid electrolytes for solid-state batteries†
Abstract
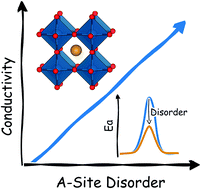
Solid electrolytes for all-solid-state batteries are generating considerable research interest as a means to improving their safety, stability and performance. Manipulation of structural disorder has a significant impact on solid electrolyte structures, but is often not fully characterised. Here, we present a comprehensive atomistic study that quantifies the effect of structural disorder on ionic transport using the perovskite Li3xLa(2/3)−xTiO3 (0 < x < 0.16) (LLTO) and its sodium analogue Na3xLa(2/3)−xTiO3 (0 < x < 0.16) (NLTO) as model solid electrolytes. We apply large-scale atomistic simulations to analyze the impact of sintering and synthesis conditions on their cation disorder and ion transport behavior. Our results predict that high temperature synthesis imparts high levels of A-site disorder in both electrolytes. The conductivities for disordered LLTO samples are consistently higher than those of ordered systems, indicating the positive correlation between disorder and Li-ion conductivity. This behavior can also be seen in NLTO, but this system suffers from very low conductivity indicating that NLTO would not be a suitable electrolyte. We discuss the role of order–disorder in the context of ionic conductivity and provide guidelines to tailor experimental synthesis conditions that can lead to the optimization of high-performance solid electrolytes.


 Please wait while we load your content...
Please wait while we load your content...