Prelithiated perfluoro-ionomer as an alternative binder for the state-of-the-art Ni-rich LiNi0.8Co0.15Al0.05O2 cathode of next-generation lithium-ion batteries†
Abstract
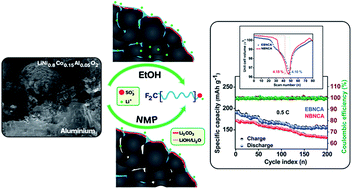
A highly flexible and ionically conductive pre-lithiated perfluoro ionomer has been employed as a promising binder for the state-of-the-art Ni-rich (LiNi0.8Co0.15Al0.05O2, NCA) cathode. Such binder has been processed in two different solvent media concerning the solubility of surface residues (in ethanol and NMP), and their influence in electrochemical performances was compared. The binder dissolved in ethanol medium displays high initial coulombic efficiency in the formation cycle, followed by superior rate retention and cycling stability due to the significant solubility of lithium residues, when compared to the NMP mediated system. The operando XRD studies reveal that both systems undergo typical anisotropic crystallite alteration during the reversible lithium extraction; however, the ethanol-mediated electrode experiences a significantly low unit-cell volume change. The differential specific capacity studies also show that the electrode processed in ethanol is less prone to voltage decay, especially at the beginning of discharge, indicating improved lithium kinetics. In addition, the diffusion coefficient is found to be 5.69 × 10−9 cm2 s−1, related to the first anodic peak.


 Please wait while we load your content...
Please wait while we load your content...