Effective hydrogen release from ammonia borane and sodium borohydride mixture through homopolar based dehydrocoupling driven by intermolecular interaction and restrained water supply†
Abstract
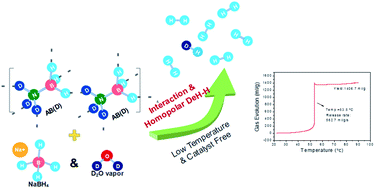
Non-catalytic dehydrogenation of solid-state ammonia borane (AB) and sodium borohydride (SB) mixtures is achieved in this work by water vapor facilitated hydrothermolysis with a maximum release rate of 560 ml g−1 s−1 at 53.5 °C and dehydrogenation capacity between 7.8 wt% (measured) and 11.7 wt% (theoretical). A novel dehydrogenation pathway is identified, in which intermolecular interaction causes the formation of intermediates and further enables the prevalence of homopolar dehydrocoupling between –BH moieties in AB and SB. The low-temperature heat used in hydrothermolysis enhances the intermolecular interaction, and the oxygen and hydrogen supplied by water vapor promote the dehydrogenation by forming products. This new hydrogen liberation method can uniquely operate without any catalyst, liquid solvent, or high temperature, and provides a compelling solution for disposable hydrogen source application. The present work also unveils for the first time the unique hydride–hydride homopolar dehydrogenation mechanism, which may unlock the true potential of chemical hydride based hydrogen storage systems.


 Please wait while we load your content...
Please wait while we load your content...