Electronic state optimization for electrochemical N2 reduction reaction in aqueous solution
Abstract
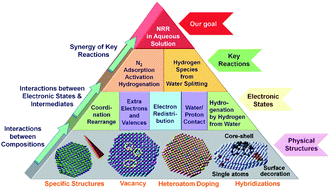
Ammonia (NH3) is an important chemical to humankind and is used in various areas as a fertilizer in agriculture, feedstock in industries and a carrier for hydrogen storage. However, the industrial NH3 production based on the Haber–Bosch (HB) method can only be done at high pressure and temperature with huge energy consumption, which makes the NH3 synthesis environmentally unsustainable. The NH3 synthesis by the electrochemical N2 reduction reaction in mild aqueous solution (e-NRR) is attractive and meaningful to overcome the shortages of the HB method, where the electrocatalysts play a critical role in the e-NRR efficiency. In particular, the electronic state is a crucial factor for the electrocatalyst to be effective in e-NRR, which is mainly determined by the elemental composition. At the same time, the physical structures (such as special morphologies), hybridization, doping, and defects (such as vacancy) can further modify the electronic state of the electrocatalyst, leading to an ideal electrocatalytic activity for e-NRR. In this review, we summarize the strategies for the electronic state optimization at the key steps in e-NRR, such as N2 adsorption, activation and hydrogenation (N2-AAH), as well as hydrogen reaction route optimization. At the end of the review, suggestions to design novel electrocatalysts and outlooks for further improvement of their catalytic performances for e-NRR are highlighted.


 Please wait while we load your content...
Please wait while we load your content...