The effect of chemically preintercalated alkali ions on the structure of layered titanates and their electrochemistry in aqueous energy storage systems†
Abstract
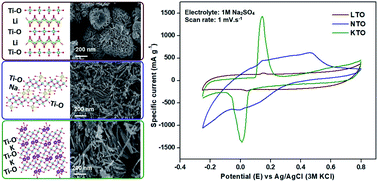
We introduce a novel chemical preintercalation synthesis technique based on a hydrogen peroxide induced sol–gel process to obtain alkali ion containing ternary layered titanates (MTO, where M = Li, Na, K). Synthesis parameters leading to the formation of single-phase materials with homogeneous elemental distribution are reported for each of the preintercalated ions. Our analyses indicate that the interlayer spacing in the structure of the layered titanates increases with the increase of the radius of the hydrated preintercalated ion. Scanning and transmission electron microscopy imaging revealed morphological diversity: the LTO phase crystallized as nanoplates assembled in “peony-like” spherical agglomerates while NTO and KTO particles exhibited a one-dimensional nanobelt or wire-like morphology, with the KTO nanobelts being shorter and more aggregated than the NTO nanobelts. Structural refinement corroborated by electron diffraction and high-resolution transmission electron microscopy revealed that the structure of the LTO phase is built by stacking Ti–O layers containing a single straight layer of connected TiO6 octahedra. The layers in NTO and KTO structures form differently and consist of double Ti–O layers with two titanium rows and ragged arrangement of units built by TiO6 octahedra. The NTO electrodes exhibited the highest electrochemical performance in cells with aqueous 1 M Na2SO4 electrolyte, followed by the KTO electrodes and then LTO electrodes, and this trend is maintained at various scan rates. The established relationships between the structure and electrochemical performance reveal that, in addition to interlayer distance and chemistry of the interlayer region, the structure of the layers can play an important role in charge storage properties of layered oxide electrodes. The double Ti–O layers in the structure of NTO and KTO phases provide a larger number of redox centers which could contribute to the superior electrochemical performance as compared to the LTO electrodes. Our findings indicate that layered materials containing double transition metal oxide layers are promising candidates for exfoliation and assembly with electronically conductive layers with the aim to create 2D heterostructures with high electrochemical performance.


 Please wait while we load your content...
Please wait while we load your content...