Revealing the perovskite formation kinetics during chemical vapour deposition†
Abstract
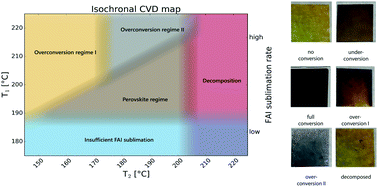
Amongst a number of deposition methods for perovskite layers, vapour based ones are promising for large area industrial production of solar cells. Different variants of such methods and high efficiencies have been reported recently, but there remains a lack of understanding on the formation process of perovskite layers with 2-step vapour deposition. Here, we present a new reactor design for a controlled investigation of the reaction kinetics for conversion of an evaporated metal halide precursor layer (such as a mixture of lead iodide and cesium bromide) into a perovskite layer by exposure to an organo-halide (such as formamidinium iodide) vapour under stable isobaric–isothermal conditions. With this new concept of gas flow reversal in a tubular reactor, we overcome an inherent problem of the lack of control over the precise start and end of the conversion process. We investigated the formation reaction of a mixed cation (Cs0.04FA0.96)PbI3 perovskite in well-defined intermediate states to elucidate the influence of processing conditions on the kinetics of perovskite and other phase formations. A high conversion rate of up to 60 nm min−1 is achieved with a well-controlled abrupt start and end of the vapor supply. Using our deposition method, a semitransparent solar cell with a power conversion efficiency (maximum power tracking) of 9.6% on a designated area of 0.27 cm2 is achieved in the initial phase of development where the charge extracting layers and interfaces are yet to be optimised.


 Please wait while we load your content...
Please wait while we load your content...