Sharp-tip enhanced catalytic CO oxidation by atomically dispersed Pt1/Pt2 on a raised graphene oxide platform†
Abstract
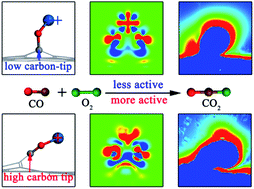
Revealing structure–activity relationships of graphene-supported atomically dispersed transition metal catalysts is of key importance in catalysis chemistry and materials science. Here we report a first-principles theoretical study on O2 activation and CO oxidation catalyzed by atomically dispersed Pt1/Pt2 anchored on a raised graphene (Gr) platform. The unique sharp-tip structure of the protuberant graphene platform (carbon-tip) can collect extra polarization electrons on the Pt-tip, promoting electron transfer between the catalyst and adsorbates, and greatly enhancing the chemical activity. Higher carbon-tips on defective graphene oxide with a carbon vacancy (Gr-V) produce more polarization electrons, a more localized electric field, and a larger up-shift of the d-orbital center of the Pt-tip, resulting in better catalytic performance. The carbon-tip enhancement reduces the energy barrier for the CO oxidation on Pt1O2/Gr-V from 1.19 eV to 0.56 eV compared with Pt1 on planar Gr. In addition, CO poisoning is significantly alleviated, with the CO poisoning rate Γθ reduced from 2.00 eV to 0.52 eV. Moreover, a linear relationship between the activation barrier and the binding energy of adsorbates is found for various atomically dispersed Pt-tip catalysts on the raised graphene oxide platform. Importantly, the order of catalytic activity is consistent with the carbon-tip enhancement, i.e., Pt1O2/Gr-V > Pt2O4/Gr-V > Pt1O2/Gr > Pt2O4/Gr. These findings provide important guidance for rational design of atomically dispersed catalysts.


 Please wait while we load your content...
Please wait while we load your content...