Nickel confined in 2D earth-abundant oxide layers for highly efficient and durable oxygen evolution catalysts†
Abstract
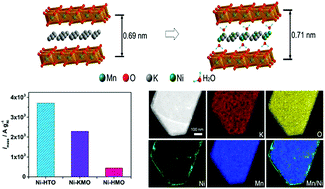
Low cost, high-efficiency catalysts towards water splitting are urgently required to fulfil the increasing demand for energy. In this work, low-loading (<20 wt%) Ni-confined in layered metal oxide anode catalysts (birnessite and lepidocrocite titanate) have been synthesized by facile ion exchange methodology and subjected to systematic electrochemical studies. It was found that Ni-intercalated on K-rich birnessite (Ni-KMO) presents an onset overpotential (ηonset) as low as 100 mV and overpotential at 10 mA cm−2 (η10) of 206 mV in pH = 14 electrolyte. By combining electrochemical methods and X-ray absorption and emission spectroscopies (XAS and XES), we demonstrate Ni sites are the active sites for OER catalysis and that the Mn3+ sites facilitate Ni intercalation during the ion-exchange process, but display no observable contribution towards OER activity. The effect of the pH and the nature of the supporting electrolyte on the electrochemical performance was also evaluated.


 Please wait while we load your content...
Please wait while we load your content...