Rational design of metal–ligands for the conversion of CH4 and CO2 to acetates: role of acids and Lewis acids†
Abstract
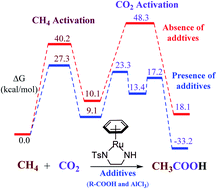
The capture, sequestration and utilization of carbon dioxide have attracted global attention in the environmental field, science and industry. The concurrent conversion of CO2 and CH4 is a reasonable solution to reduce greenhouse gases, but it remains a challenging research topic. Herein, we reveal the catalytic activity of bifunctional half-sandwich complexes (metal = Ru, Rh, and Ir) in the direct conversion of methane and carbon dioxide to acetic acid (AA) using density functional theory. The use of Ru/Rh/Ir metals with ethanediphosphine (PP), N-tosylethylenediamine (NNTs), and ethylene glycol (OO) ligands shows superb performance in lowering the free energy (ΔG) barrier (ΔG‡) for the conversion. To activate CH4 and CO2 molecules, we used extra additives to reduce the high energy barriers required by simple metal–ligand complexes. For CH4 activation, additives of AA and trifluoroacetic acid (TFA) were used to assist the proton abstraction in the Ru–NNTs/OO complexes, while no acid additives were used for the PP ligands. In the case of the PP ligands, the C–H activation occurs through a “concerted oxidative addition–reductive elimination” (OA–RE) process, whereas in the NNTs/OO ligands, C–H activation occurs through “cyclometallation deprotonation” (CMD) irrespective of the presence of acid additives. The reduction in ΔG‡ for CH4 activation in Ru–NNTs/OO is 5–10 kcal mol−1 using acid additives. In contrast, in Ru–PP, this ΔG‡ barrier is 20 kcal mol−1 without additives. For CO2 activation, AlCl3 (Lewis acid) was used to insert CO2 into the metal site of the Ru–NNTs/OO and Ru/Rh/Ir–PP complexes. The reduction in ΔG‡ using AlCl3 for CO2 activation in Ru–NNTs·AA/OO·2TFA and Ru/Rh/Ir–PP is 15–30 kcal mol−1. Then, the overall activation barrier is reduced to 20–27 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...