Ultralow thermal conductivity and high thermoelectric figure of merit in mixed valence In5X5Br (X = S, and Se) compounds†
Abstract
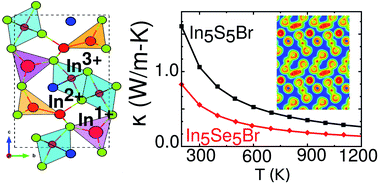
Discovering a material with a combination of high electrical conductivity, high thermopower, and low lattice thermal conductivity is crucial for designing efficient thermoelectric materials. Here, we show that a perfect balance of these properties can be realized in In5X5Br (X = S and Se) compounds, where indium simultaneously exists in three different oxidation states (In1+, In2+ and In3+). The presence of multiple charge carrier pockets near the band edge results in a high thermopower of 250–300 μV K−1 for both p- and n-type doping over a wide range of carrier concentrations and temperatures. Furthermore, our calculations find an exceptionally low lattice thermal conductivity of 0.55 W m−1 K−1 and 1.1 W m−1 K−1 for In5Se5Br and In5S5Br at room temperature, respectively. This ultralow lattice thermal conductivity is attributed to In1+, which is very weakly bound in the lattice. These In1+ atoms are located in a flat potential well and exhibit large mean square displacements demonstrating the strong anharmonic behavior. The strong anharmonicity was further confirmed by the large negative Grüneisen parameters. Remarkably, these compounds possess a rare combination of low thermal conductivity and high thermopower leading to an exceptionally high ZT of ∼2.4 and 2.7 for n-type In5S5Br and In5Se5Br, respectively, at high temperatures. The insights obtained from this work suggest an opportunity to discover efficient thermoelectric materials among other unexplored mixed valence compounds.


 Please wait while we load your content...
Please wait while we load your content...