Au nanoparticle-embedded, nitrogen-deficient hollow mesoporous carbon nitride spheres for nitrogen photofixation†
Abstract
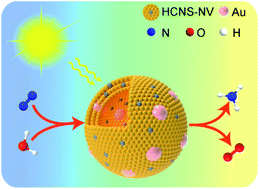
Ammonia, one of the most important chemicals and an efficient energy carrier, is industrially synthesized by the energy-intensive Haber–Bosch process. Photocatalytic nitrogen fixation under ambient conditions provides an intriguing approach for the conversion of atmospheric dinitrogen into ammonia. Herein we report a plasmonic hybrid catalyst composed of Au nanoparticles uniformly embedded in the mesopores of nitrogen-deficient hollow carbon nitride spheres for efficient nitrogen photofixation. The nitrogen vacancies in the carbon nitride spheres, serving as the sites for nitrogen chemisorption and activation, capture photoexcited electrons originating from the carbon nitride spheres and the plasmon resonance of the embedded Au nanoparticles for the reduction of nitrogen to ammonia. The designed structure can maximize the utilization efficiency of the plasmonic effect of the Au nanoparticles, as well as the nitrogen activation effect of the nitrogen vacancies in the carbon nitride spheres, therefore promoting the photoreduction of nitrogen. The optima Au-embedded carbon nitride spheres achieve an ammonia production rate of 783.4 μmol h−1 gcat−1 under visible light. The interfacial plasmon-induced charge separation endows the hybrid photocatalyst with the capability of simultaneous production of ammonia and oxygen with a solar-to-ammonia conversion efficiency of 0.032% under simulated sunlight.
- This article is part of the themed collection: Journal of Materials Chemistry A Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...