Probing electrochemical reactivity in an Sb2S3-containing potassium-ion battery anode: observation of an increased capacity†
Abstract
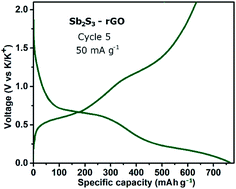
Potassium-ion batteries are attracting considerable attention as a viable type of high voltage battery. Among available anode materials, composites containing Sb2S3 are some of the most interesting high capacity candidates. A nanostructured Sb2S3–reduced graphene oxide composite anode material is evaluated in this study and compared with a structurally similar SnS2–reduced graphene oxide material reported previously by this team. The behaviour of the Sb2S3-based electrodes is assessed in both 1 M KPF6 in ethylene carbonate–diethyl carbonate and 1 M KPF6 in 1,2-dimethoxyethane electrolytes. Depotassiation capacities in excess of 650 mA h g−1 are recorded for the composite electrodes, superior not only to SnS2-based electrodes but also to all previously reported Sb2S3-containing electrode materials for potassium-ion batteries. In order to establish insights into the reaction mechanism of the Sb2S3 phase with potassium, post-cycling X-ray diffraction and in situ transmission electron microscopy are utilised. The recorded data suggest the presence of antimony alloys and potassium polysulphides as reaction products and intermediates; a possible conversion-alloying reaction mechanism is discussed. The results indicate that a capacity higher than previously believed is achievable in the Sb2S3 active component of potassium-ion battery electrodes.


 Please wait while we load your content...
Please wait while we load your content...