On competitive gas adsorption and absorption phenomena in thin films of ionic liquids†
Abstract
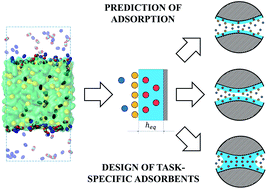
Although there has been a lot of interest in materials that feature thin films of ionic liquids on the surface of porous materials, fundamental understanding of gas–liquid interfacial processes is still lacking, hindering the development of novel adsorbents and adsorption models for practical applications. Herein, we investigated the mechanism of competitive gas adsorption on and absorption in thin films of ionic liquid, [BMIM]+[PF6]−, exposed to the gas phase containing carbon dioxide and nitrogen. To estimate correct quantitative contributions of these processes, we performed classical molecular dynamics simulations of the gas–liquid interfacial systems. Adsorption of gases proceeds through the formation of an adsorbed gas layer on the surface of the ionic liquid and partial dissolution of the gas in the bulk liquid phase. To characterize the competition between these two processes we introduced a parameter, the equipartition thickness of the film of ionic liquid, which relates the contributions of gas dissolved in the liquid phase and gas adsorbed on the surface of the film to the total amount adsorbed. At a given temperature, the equipartition thickness is constant for a specific gas-ionic liquid pair in the Henry's law regime, where uptake is proportional to the applied gas pressure. Through the combination of computational and available experimental studies, we propose how a single property, the equipartition thickness, may govern the development of task-specific porous materials and predict their performance as well as thermodynamics of gas adsorption.


 Please wait while we load your content...
Please wait while we load your content...