Activating the lattice oxygen in (Bi0.5Co0.5)2O3 by vacancy modulation for efficient electrochemical water oxidation†
Abstract
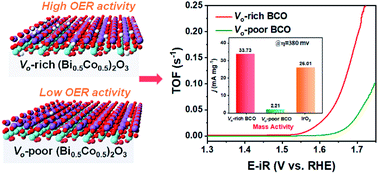
The catalytic activity for the oxygen evolution reaction (OER) in electrocatalytic water splitting strongly depends on the adsorption energy of intermediates. For the generally proposed adsorbate evolution route, the universal scaling relation between the adsorption energies of *OOH and *OH leads to an OER efficiency limitation based on the “volcano curve”. A possible solution to bypass the scaling relation is to avoid the formation of the *OOH intermediate in the OER with the participation of lattice oxygen from catalysts. In this work, the lattice oxygen in (Bi0.5Co0.5)2O3 is activated through adjusting the Fermi energy level and the strong overlap between Co 3d and O 2p, by means of increasing the oxygen vacancy concentration. Compared to oxygen-vacancy-poor (Bi0.5Co0.5)2O3, the oxygen-vacancy-rich (Bi0.5Co0.5)2O3 exhibits a significantly lower Tafel slope (43 mV dec−1), 15 times higher mass activity, 18 times higher turnover frequency, and excellent long-term stability in alkaline media, superior to those of the benchmark OER electrocatalyst IrO2. This work provides a feasible strategy to activate lattice oxygen with fast OER kinetics and puts forward the development of efficient and stable catalysts towards water oxidation.


 Please wait while we load your content...
Please wait while we load your content...