Decoupled electrolytes towards enhanced energy and high temperature performance of thermally regenerative ammonia batteries†
Abstract
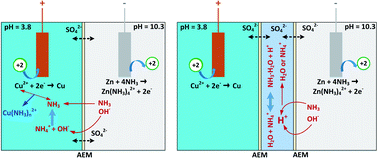
Thermally regenerative ammonia batteries (TRABs) show viable potential for harvesting abundant low-grade waste heat as high-power electricity. However, the ammonia brings about a large pH difference between the catholyte and anolyte, leading to self-discharge and severe energy decay. Here, an electrolyte decoupling strategy is proposed for ammonia batteries to restrain the self-discharge and enhance energy density as well as power generation at high temperatures. The self-discharge by ion cross contamination is observed visually as a colour evolution of the interlayer solution, and a transition of the principal cathodic reactant from Cu2+ to Cu(NH3)42+ exists during discharging, which signals the beginning of performance degradation. The results demonstrate that decoupled Cu/Zn-TRABs with double and triple-membrane designs improve the energy density by 45–50%, mainly due to the delay of the transition region. The power density of the decoupled Cu/Zn-TRABs is reduced at high currents, but with concentration optimization or elevated temperatures, it is able to be promoted significantly. With an energy density of 1034 W h m−3 obtained by a decoupled Cu/Zn-TRAB with double-IEM (ion exchange membrane) design, a thermoelectric conversion efficiency of 1.86% (15.6% relative to the Carnot efficiency) is achieved at a condenser temperature of 16 °C.


 Please wait while we load your content...
Please wait while we load your content...