Effects of linking units on fused-ring electron acceptor dimers†
Abstract
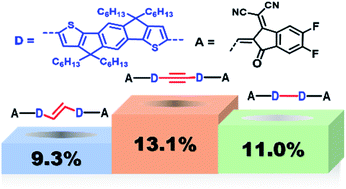
Three fused-ring electron acceptors (SIDIC, DIDIC and TIDIC) were designed and synthesized using single bond, vinylene and acetylene units linked indaceno[3,2-b]dithiophene dimers as electron-rich cores and 3-(1,1-dicyanomethylene)-5,6-difluoro-1-indanone as electron-deficient termini. These molecules exhibit strong absorption from 550 to 900 nm with large attenuation coefficients of 1.8–2.0 × 105 M−1 cm−1 and high electron mobilities of 2.2–4.9 × 10−3 cm2 V−1 s−1. In combination with wide-bandgap polymer FTAZ as a donor, organic solar cells exhibit efficiencies of 9.3–13.1%. Effects of the linking units on optical, electronic, morphologic, and photovoltaic properties were revealed. Relative to SIDIC, vinylene-bridged DIDIC shows red-shifted absorption, while acetylene-bridged TIDIC shows blue-shifted absorption. Compared with SIDIC and DIDIC, TIDIC has a lower HOMO, higher electron mobility, and higher device efficiency.


 Please wait while we load your content...
Please wait while we load your content...