High performance multicomponent bifunctional catalysts for overall water splitting†
Abstract
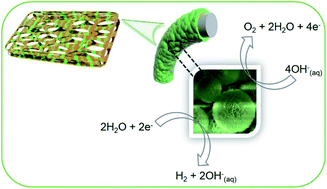
Designing highly active bifunctional electrocatalysts from Earth-abundant elements has great prospects for substituting precious-metal based catalysts in energy conversion processes, such as water splitting. Here, we report a bifunctional catalyst comprising transition metal hydroxides (TMOHs) and transition metal sulphides (TMSs) grown on a nickel foam (NF) surface, denoted as NiFeOH/CoSx/NF, that delivers high electrocatalytic activity for both the oxygen evolution reaction (OER: ultra-low overpotential of 211 mV at a current density of 50 mA cm−2) and the hydrogen evolution reaction (HER: overpotential of 146 mV at a current density of 10 mA cm−2) in alkaline media, representing one of the best bifunctional catalytic performances yet reported for a non-noble metal based system. From our experimental observations, the significant improvement of the catalytic activity emanates from the synergistic effects of NiFeOH and CoSx, due to the optimization of their electronic configurations, thereby creating novel characteristics. Employing this catalyst system as both the anode and the cathode for overall water splitting in a water electrolyzer delivers 10 mA cm−2 at a low cell potential of 1.563 V with excellent long-term electrocatalytic functionalities over 10 h of continuous operation. These findings represent the design principle for developing multi-component bifunctional electrocatalysts for overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...