Molecular-level environments of intercalated chloroaluminate anions in rechargeable aluminum-graphite batteries revealed by solid-state NMR spectroscopy†
Abstract
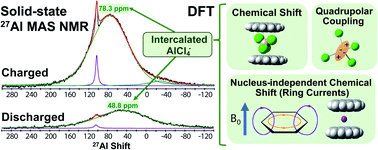
Rechargeable aluminum–graphite batteries are an emerging energy storage technology with great promise: they exhibit high rate performance, cyclability, and a discharge potential of approximately 2 V, while both electrodes are globally abundant, low cost, and inherently safe. The batteries use chloroaluminate-containing electrolytes and store charge in the graphite electrodes when molecular AlCl4− anions electrochemically intercalate within them. However, much remains to be understood regarding the ion intercalation mechanism, in part due to challenges associated with characterizing the chloroaluminate anions themselves. Here, we use solid-state 27Al nuclear magnetic resonance (NMR) spectroscopy to probe the molecular-level electronic and magnetic environments of intercalated chloroaluminate anions at different states-of-charge. The results reveal broad 27Al NMR signals associated with intercalated AlCl4− anions, reflecting high extents of local disorder. The intercalated anions experience a diversity of local environments, many of which are far from the ideal crystalline-like structures often depicted in graphite staging models. Density functional theory (DFT) calculations of the total 27Al isotropic shifts enable the contributions of chemical shift, ring-current effects, and electric quadrupolar interactions to be disentangled quantitatively. In combination, the solid-state NMR and DFT results reveal the molecular geometries and environments of intercalated AlCl4− anions and capture the significant disorder present in intercalated graphite battery electrodes.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...