Enabling electrochemical N2 reduction to NH3 in the low overpotential region using non-noble metal Bi electrodes via surface composition modification†
Abstract
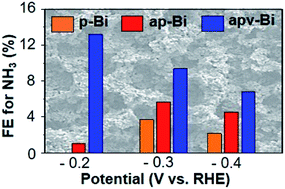
The electrochemical N2 reduction reaction (ENRR) that can produce NH3 using water as the hydrogen source at ambient temperature and pressure can be an exciting alternative to the Haber–Bosch process. The major challenge for electrochemical NH3 production is the competing hydrogen evolution reaction (HER), which seriously limits the faradaic efficiency (FE) for NH3 production. To date, noble metal electrocatalysts that are inactive for the HER have mainly been investigated for the ENRR. Studies reporting a FE greater than 10% for NH3 production using non-noble metal catalysts in the low overpotential region (E ≤ 0.2 V vs. RHE) are very rare. This study reports effective electrochemical surface modification strategies that drastically increase the ENRR activity of a non-noble Bi electrode in the low overpotential region and achieve a FE for NH3 production as high as 13.2% at −0.2 V vs. RHE in pH 7.5 phosphate buffer. The effect of each of the surface modifications on the activity for the ENRR and the electrode stability during the ENRR were systematically elucidated, which may be used to develop general strategies to enhance the ENRR activities of other non-noble metal electrodes in the low overpotential region.


 Please wait while we load your content...
Please wait while we load your content...