Interphase-oxidized ruthenium metal with half-filled d-orbitals for hydrogen oxidation in an alkaline solution†
Abstract
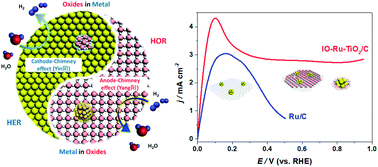
Ruthenium is generally used as a property-promoter rather than as the main catalyst for the hydrogen oxidation reaction (HOR) because of the preferential formation of Ru–O(H) at a relatively low anode potential (0.1–0.3 V vs. the reversible hydrogen electrode, RHE), which leads to few oxide-free Ru atoms remaining for bonding to H intermediates. Here, we report an uncommon finding that sub-2 nm Ru clusters can efficiently catalyze the HOR at a potential of up to 0.9 V (vs. RHE) without being oxidized, when the Ru clusters are interphase-oxidized by TiO2 to yield nearly half-filled 4d-orbitals (IO-Ru–TiO2/C). The X-ray absorption and X-ray photoelectron spectroscopy results confirmed that the Ru clusters in IO-Ru–TiO2/C are preoxidized close to a level of RuIV and maintain a metallic surface with abundant metallic Ru–Ru bonds. These features decrease the adsorption of oxygen species during the HOR and greatly promote HOR activity and inoxidizability under alkaline conditions. As a result, a mass activity of 907 A g−1 Ru @ 0.05 V is achieved for the IO-Ru–TiO2/C catalyst, which is 17.5 and 1.5 times greater than those of Ru/C and PtRu/C catalysts, respectively, ranking it among the most active HOR catalysts in alkaline media reported to date.


 Please wait while we load your content...
Please wait while we load your content...