Enhanced electrocatalytic hydrogen evolution on a plasmonic electrode: the importance of the Ti/TiO2 adhesion layer†
Abstract
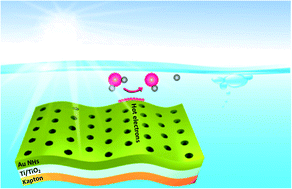
The electrochemical production of hydrogen is enjoying renewed vigor due to its great promise as an environmentally friendly energy alternative. Herein, we demonstrate the capacity of nanoperforated gold films deposited on flexible substrates coated with nanometer thick Ti/TiO2 adhesion layers for electrochemical water splitting under light irradiation. The strong plasmonic induced electromagnetic field enhancement, which occurs under the illumination of plasmonic electrodes, facilitates the dissociation of water into hydrogen and improves the electrocatalytic hydrogen evolution activity beyond that of platinum. Under illumination at 980 nm at 2 W cm−2, a current density of −10 mA cm−2 was recorded at a low overpotential of η ≈ −0.045 V (vs. RHE); under the same conditions, a Pt electrode achieved −10 mA cm−2 at η ≈ −0.048 V (vs. RHE). Mechanistic investigations revealed that plasmon-excited perforated gold thin layers are an efficient source of hot electrons, contributing to their injection into the underlying TiO2-based adhesion layer. Direct electrochemical reduction of water appears to be a parallel reaction pathway to sustain the hydrogen evolution reaction on the interface. Both reaction pathways decrease the activation energy for the hydrogen evolution reaction similar to the benchmark Pt electrocatalyst.


 Please wait while we load your content...
Please wait while we load your content...