Electrochemical conversion of bulk platinum into platinum single-atom sites for the hydrogen evolution reaction†
Abstract
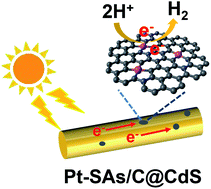
Herein, we report a convenient and controllable electroplating strategy to fabricate single-atom site catalysts directly from bulk metals with the assistance of graphene oxide membrane (GOM) under mild and ambient conditions. With the regulation of the GOM, the diffusion rate and the concentration of metal ions around the working electrode are efficiently decreased; consequently, the growth of nuclei and aggregation of atoms are successfully prevented. The synthesized Pt-SAs/C not only exhibits an outstanding electrocatalytic activity towards the hydrogen evolution reaction (HER), but also an excellent photocatalytic activity for water splitting with CdS as the photosensitizer under visible-light irradiation. This work provides an efficient strategy for developing single-atom site catalysts towards hydrogen production.


 Please wait while we load your content...
Please wait while we load your content...