Unexpected electrochemical behavior of an anolyte redoxmer in flow battery electrolytes: solvating cations help to fight against the thermodynamic–kinetic dilemma†
Abstract
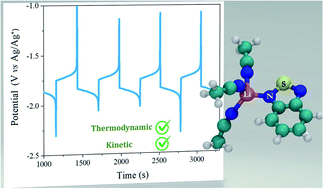
Redoxmers are redox-active molecules that can store energy in electrolytes for redox flow batteries (RFBs), and their electrochemical properties are significantly affected by the choice of supporting electrolytes. Herein, we use 2,1,3-benzothiadiazole (BzNSN) as a model system to scrutinize the supporting electrolyte impact. By systemically varying the components of supporting salts, BzNSN not only shows substantial redox potential shifts but also exhibits varying electrochemical stabilities. Specifically, changing the size of cations can effectively alter the coordination between the supporting salt and BzNSN species. From Li+, Na+, K+, to NEt4+, the redox potential of BzNSN shifts negatively, from −1.63 V to −1.82 V vs. Ag/Ag+. Molecular dynamics and density functional theory simulations revealed that smaller cations, like Li+, are closer to the charged BzNSN when coordinated, implying stronger coordination, while larger cations, like K+ and NEt4+, are farther away. Interestingly, the large cation electrolytes also lead to much improved electrochemical stability, evidenced by the extraordinarily enhanced kinetic lifetime from electron paramagnetic resonance measurement. This study demonstrates the first example of tuning an anolyte redoxmer toward a concurrent improvement of lowered redox potentials AND enhanced calendar lives via solvation means, which is usually constrained by the thermodynamic–kinetic relation.


 Please wait while we load your content...
Please wait while we load your content...
