Modulating reactivity and stability of metallic lithium via atomic doping†
Abstract
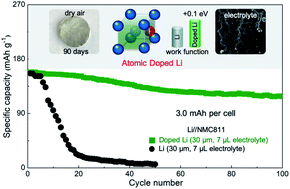
Many approaches have been described to address challenges in metallic Li anodes; but they rarely modulate the inherent chemical reactivities. Here we describe a general approach for modulating Li metal properties and enabling stable metal batteries by doping with ∼0.1 at% Ag or Al. The dopants were atomically dispersed in the vacant face-centered sites of the body-centered cubic Li crystals and pull electrons strongly due to higher electronegativity (Ag: 1.98; Al: 1.61 vs. Li: 0.98). As a result, the doped Li anodes have increased work function with reduced chemical reactivity and remained shiny in dry air for months. They also exhibited enhanced Li+/Li redox kinetics and generated thinner but stronger solid-electrolyte interphases in carbonate electrolytes. The dopant atoms are lithophilic and have stronger binding with Li adatoms, which guide uniform Li deposition and ensures dendrite-free Li interface during battery cycling. Overall, the doped anodes enabled stable operations of not only high current symmetric cells but also practical full cells in which Ni-rich layered cathodes were paired with 30 μm anodes and 7 μL electrolytes. The doping approach is facile and scalable, and opens up new and promising opportunities for designing practical high energy density metal batteries.


 Please wait while we load your content...
Please wait while we load your content...