A robust ionic liquid magnesium electrolyte enabling Mg/S batteries†
Abstract
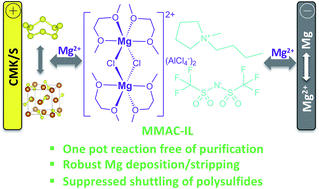
Mg batteries are attractive for low-cost and sustainable energy storage because Mg as an anode material is highly abundant in the crust of the Earth, it has a high charge capacity (2205 Ah kg−1 or 3832 A h L−1) benefiting from its two electron redox chemistry, and it undergoes less dendritic growth than other metals (Li and Na). However, the development of Mg batteries is still hampered by the lack of easy-to-make, chemically stable Mg electrolytes. In the current work, we conveniently prepared an ionic liquid Mg electrolyte (MMAC-IL) by mixing MgCl2, AlCl3, Mg, and 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide (Pyr14TFSI) together and using them in a one-pot reaction; the product of this reaction did not need to be purified. The MMAC-IL electrolyte exhibited superior anodic and cathodic electrochemical performances, according to electrochemical and spectroscopic studies. Specifically, MMAC-IL delivered robust Mg deposition/stripping cycling for 300 h with minimum polarization change in Mg/Mg half-cell studies. Moreover, the MMAC-IL electrolyte with its high ionic strength significantly suppressed the shuttle effect of magnesium polysulfides and enabled a stable Mg–S battery.


 Please wait while we load your content...
Please wait while we load your content...