A thermodynamically stable quasi-liquid interface for dendrite-free sodium metal anodes†
Abstract
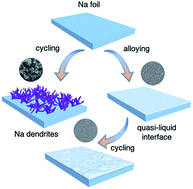
Dendrite-associated cell degradation is a key challenge to the practical application of sodium metal anodes. Here, we present that incorporation of a tiny amount of mercury into sodium generates a unique quasi-liquid interface that affords long-term cycling stability. This amalgam layer allows fast electron transfer and sodium migration at the electrolyte–electrode interphase, which significantly promote the cycling performance over 5000 h with a practically desired capacity of 2 mA h cm−2 and a current density of 8 mA cm−2. In situ optical microscopy analyses confirm that dendrite nucleation and growth can be remarkably suppressed with the amalgam-protected anodes. Prototype full cells also demonstrate a much improved rate and long-term cycling stability. These promising results provide new perspectives on the regulation of sodium electrodeposition by introducing low-melting metals and hence the elimination of the dendritic morphology for the practical development of sodium metal batteries.


 Please wait while we load your content...
Please wait while we load your content...