The HER/OER mechanistic study of an FeCoNi-based electrocatalyst for alkaline water splitting†
Abstract
Herein, the electrodeposited-film electrode CFeCoNiP was fabricated to serve as a bifunctional electrocatalyst for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Kinetic Tafel slope analysis suggests that the HER follows the Volmer–Tafel mechanism (29 mV dec−1), indicating that the recombination of the two adsorbed hydrogen atoms is the rate-determining step. The FeCoNi-based thick film (thickness: 168.3 μm) shows a metallic state favorable for electron transfer; on the other hand, in the case of the FeCoNi-based thin film (thickness: 389.2 nm), the in operando XAS investigation reveals that Fe3+-assisted water dissociation promotes the formation of Co2+–μ-H–Ni3+ (catalyst-Had) species, which subsequently undergoes reductive elimination to furnish H2 gas via the HER process. During the OER, the CoNi–oxide matrix acts as a chemical and electroconductive host to build/stabilize the key intermediate [Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Fe3+–O˙] motifs; this subsequently triggers the catalytic O–O bond formation (30 mV dec−1) through the radical–radical coupling of the adjacent [Fe4+
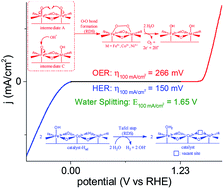
O/Fe3+–O˙] motifs; this subsequently triggers the catalytic O–O bond formation (30 mV dec−1) through the radical–radical coupling of the adjacent [Fe4+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Fe3+–O˙] motifs or/and OH− attack on the Fe4+-induced electrophilic oxygen center, leading to the release of O2. The mechanistic experiments provide advanced insights into the catalytic kinetics/intermediates and demonstrate that the electronically cooperative interplay among Fe/Co/Ni leads to enhanced alkaline water electrolysis. The CFeCoNiP catalyst exhibits an excellent HER activity (specific activity js = 0.227 mA cm−2) with a low charge transfer resistance (3.9 Ω) and an overpotential of 37 mV, achieving the current density of 10 mA cm2; moreover, it shows good OER activity (js = 1.798 mA cm−2) with low charge transfer resistance (2.1 Ω) and an overpotential of 250 mV, approaching a current density of 10 mA cm−2 in a 1 M NaOH aqueous solution. The CFeCoNiP/NF (electrodeposited on Ni foam) electrode-pair device achieved the current densities of 100 and 500 mA cm−2 at the voltages of 1.65 and 1.86 V, respectively, under alkaline conditions.
O/Fe3+–O˙] motifs or/and OH− attack on the Fe4+-induced electrophilic oxygen center, leading to the release of O2. The mechanistic experiments provide advanced insights into the catalytic kinetics/intermediates and demonstrate that the electronically cooperative interplay among Fe/Co/Ni leads to enhanced alkaline water electrolysis. The CFeCoNiP catalyst exhibits an excellent HER activity (specific activity js = 0.227 mA cm−2) with a low charge transfer resistance (3.9 Ω) and an overpotential of 37 mV, achieving the current density of 10 mA cm2; moreover, it shows good OER activity (js = 1.798 mA cm−2) with low charge transfer resistance (2.1 Ω) and an overpotential of 250 mV, approaching a current density of 10 mA cm−2 in a 1 M NaOH aqueous solution. The CFeCoNiP/NF (electrodeposited on Ni foam) electrode-pair device achieved the current densities of 100 and 500 mA cm−2 at the voltages of 1.65 and 1.86 V, respectively, under alkaline conditions.
- This article is part of the themed collections: Energy Frontiers: Hydrogen and Journal of Materials Chemistry A Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...