Chemoselective hydrogenation of α,β-unsaturated aldehydes over Rh nanoclusters confined in a metal–organic framework†
Abstract
Selective hydrogenation of α,β-unsaturated aldehydes to achieve high selectivity towards a desirable product is still a great challenge mainly because of the complex conjugate system. Herein, Rh nanoclusters encapsulated in MIL-101 (Cr), synthesized by the double solvent method, are able to selectively hydrogenate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of cinnamaldehyde, an α,β-unsaturated aldehyde and achieve over 98% selectivity with a conversion of 98% to a saturated aldehyde under mild conditions. Fourier transform infrared spectroscopy confirms that MIL-101 acts as an aldehyde protector to suppress the reactivity of C
C of cinnamaldehyde, an α,β-unsaturated aldehyde and achieve over 98% selectivity with a conversion of 98% to a saturated aldehyde under mild conditions. Fourier transform infrared spectroscopy confirms that MIL-101 acts as an aldehyde protector to suppress the reactivity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the X-ray photoelectron spectroscopy (XPS) data indicate that the electropositive Rh, owing to the electron transfer from Rh to MIL-101, preferentially absorbs C
O, and the X-ray photoelectron spectroscopy (XPS) data indicate that the electropositive Rh, owing to the electron transfer from Rh to MIL-101, preferentially absorbs C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C rather than C
C rather than C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O leading to improvement of the selectivity towards saturated aldehydes. In addition, Rh@MIL-101 can also efficiently catalyse hydrodefluorination of aryl fluorides with good stability. This work provides a basic strategy to develop other selective heterogeneous catalysts via structural modulation for synergetic catalysis.
O leading to improvement of the selectivity towards saturated aldehydes. In addition, Rh@MIL-101 can also efficiently catalyse hydrodefluorination of aryl fluorides with good stability. This work provides a basic strategy to develop other selective heterogeneous catalysts via structural modulation for synergetic catalysis.
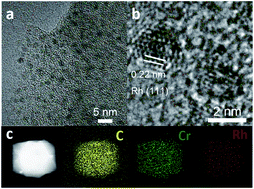


 Please wait while we load your content...
Please wait while we load your content...