Controllable electrolytic formation of Ti2O as an efficient sulfur host in lithium–sulfur (Li–S) batteries†
Abstract
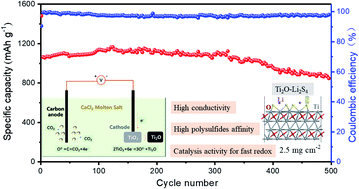
The insulation of S/Li2S and highly soluble nature of polysulfides largely limit the practical use of Li–S batteries. To overcome these issues, tremendous effort has been made for exploring efficient host materials and new battery construction. Herein, we propose to prepare high conductivity Ti2O by a molten salt electrochemical synthesis method; the synthesized Ti2O is developed to serve as an advanced host and used as a functional separator modification material for the first time. XPS analysis and DFT calculations verified that Ti2O can chemically anchor polysulfides through the abundant surface vacancies, and its high electronic conductivity can further enhance the redox kinetics of intermediate polysulfides. The cells based on the Ti2O–Super P/S cathode exhibit a high sulfur utilization and superb rate capability (553 mA h g−1 at 4C). Moreover, the cells assembled with a host-interlayer integrated electrode (2.5 mg cm−2) could achieve a low decay rate of 0.04% over 500 cycles at 0.5C. A high areal capacity of 6.8 mA h cm−2 can be also achieved even under 7.5 mg cm−2 sulfur loading. Overall, the controllable synthesis and application strategies of Ti2O present a way of regulation and optimization of the electrochemical performance of a Ti-based sulfur host through surface vacancy engineering for advanced Li–S batteries.


 Please wait while we load your content...
Please wait while we load your content...