Naphthalene diimide as a two-electron anolyte for aqueous and neutral pH redox flow batteries†
Abstract
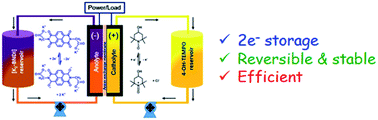
Developing organic materials that show stable and highly reversible redox-activity is a key challenge for achieving cost-effective and sustainable aqueous redox flow batteries (RFBs) for practical energy storage applications. We report a water-soluble naphthalene diimide (NDI) derivative using carboxylate groups, which was tested and established as a new class of versatile anolytes capable of storing two electrons in a single molecule in aqueous and neutral pH RFBs. The potassium salt of N,N′-bis(glycinyl)naphthalene diimide [K2-BNDI] showed reversible and stable two-electron reductions accompanied by ion- pairing. A prototype aqueous RFB consisting of [K2-BNDI] and 4-OH-TEMPO was constructed and showed excellent cyclability, high energy and voltage efficiencies. This study suggests a versatile way of designing new anolytes inspired by organic semiconductors that operate in a multi-electron redox mode.


 Please wait while we load your content...
Please wait while we load your content...