Long-term electrocatalytic N2 fixation by MOF-derived Y-stabilized ZrO2: insight into the deactivation mechanism†
Abstract
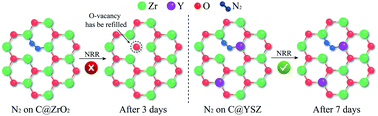
Industrially, NH3 synthesis is largely dependent on the Haber–Bosch method which consumes a lot of energy and emits huge amounts of CO2. Recently, the electrochemical N2 reduction reaction (NRR) has been recognized as a promising method to achieve clean and sustainable NH3 production, thus highly efficient and durable catalysts are urgently desired. In this paper, we report a MOF-derived carbon/Y-stabilized ZrO2 nanocomposite (C@YSZ) that works as an efficient electrocatalyst for NRR in 0.1 M Na2SO4. It achieves a large NH3 production of 24.6 μg h−1 mgcat.−1 and a high faradaic efficiency of 8.2% at −0.5 V vs. the reversible hydrogen electrode. The experimental results demonstrate that the surface oxygen vacancies are the main catalytic sites for NRR. Introducing Y3+ into the ZrO2 lattice has a significant effect to increase and stabilize the O-vacancies. Meanwhile, this catalyst displays remarkable stability and durability for NRR, showing negligible change after 7 days reaction, which is better than most reported NRR electrocatalysts. Moreover, an in situ electrochemical quartz-crystal microbalance (EQCM) was applied in the NRR field for the first time and was successfully combined with density functional theory (DFT) calculations to reveal the deactivation mechanism.


 Please wait while we load your content...
Please wait while we load your content...