Aliovalent fluorine doping and anodization-induced amorphization enable bifunctional catalysts for efficient water splitting†
Abstract
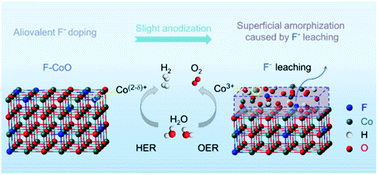
High-efficiency non-precious electrocatalysts are urgently demanded in the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Transition metal oxide catalysts have shown high activity for the OER in alkaline medium, however, they are generally inactive for the HER. Here, we report an aliovalent fluorine anion substitution and anodization-induced amorphization strategy to enhance the HER activity of CoO and concomitantly obtain the optimal OER performance. Aliovalent fluorine anions at O2− sites significantly induce the charge redistribution of neighboring Co sites, facilitate water dissociation and weaken hydrogen adsorption, enabling the optimal states for the HER. More importantly, the anodization causes F-leaching-assisted amorphization, which leads to the formation of a highly catalytic shell for the OER. When using F-CoO as the bifunctional catalyst, a low cell voltage of 1.53 V is observed to drive a current density of 10 mA cm−2, which is superior to that of noble-metal-based Pt/C–IrO2. This work provides a novel strategy of aliovalent fluorine anion substitution to dramatically activate transition metal oxides for overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...