Intermolecular electron modulation by P/O bridging in an IrO2-CoPi catalyst to enhance the hydrogen evolution reaction†
Abstract
Development of a highly efficient electrocatalyst for the hydrogen evolution reaction (HER) remains a great challenge. Herein, a catalytic system is constructed based on a low content of atomically dispersed iridium oxide (IrO2) coordinated with cobalt phosphate (CoPi) onto carbon nanotubes (CNTs) by a two-step electrochemical deposition procedure. The resultant IrO2-CoPi-CNT catalytic system with a low loading of Ir (0.41 wt%) has Pt-like performances with a small overpotential of 29 mV at a current density of 10 mA cm−2 and a low Tafel slope of 27 mV dec−1 towards the HER. X-ray photoelectron spectroscopy (XPS) measurements and density functional theory (DFT) calculations reveal that the electronic structure of CoPi and IrO2 can be effectively modulated through electron-donating phosphate bonds (P/O bridging),thus giving a more favorable ΔGH* with a value of −0.13 eV. These bonds also prevent IrO2 from undergoing further aggregation. Besides, CoPi can rapidly cleave HO–H bonds and transfer an abundance of H* intermediates to nearby IrO2 catalytic sites, facilitating the formation and release of H2 molecules. This work manifests the importance of the electron structure of catalysts in electrocatalysis and provides an avenue for preparing highly active electrocatalysts towards the HER.
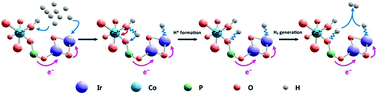


 Please wait while we load your content...
Please wait while we load your content...