Thermoelectrochemical formation of Fe/Fe3C@hollow N-doped carbon in molten salts for enhanced catalysis†
Abstract
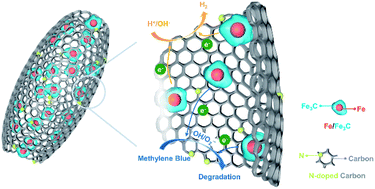
The interaction between iron and non-metals is a classic topic in industrial iron/steel making and catalysis, in which the modulation of the linkage and distribution of iron and non-metals is a key factor to develop advanced Fe-based materials. Herein, a hybrid of Fe/Fe3C nanoparticles strongly encapsulated in a nitrogen-doped carbon (N–C) hollow shell is prepared through the thermoelectrochemical treatment of Fe2O3@polydopamine (Fe2O3@PDA) in molten NaCl–CaCl2 at 600 °C. The coupling of the thermal pyrolysis of PDA and electrochemical reduction of Fe2O3 contributes to generating Fe/Fe3C species at a relatively low temperature, ensuring structural integrity and in situ N doping for the hybrid to achieve a well-defined hollow structure and abundant active sites of Fe–N and C–N species. The hollow carbon skeleton guarantees efficient mass transfer and maximum exposure of active sites. Consequently, Fe/Fe3C@N–C exhibits appealing activity for electrocatalytic hydrogen evolution and Fenton-like reaction and importantly, it can be readily separated for reuse by a magnetic field.


 Please wait while we load your content...
Please wait while we load your content...