Electronic band contraction induced low temperature methane activation on metal alloys†
Abstract
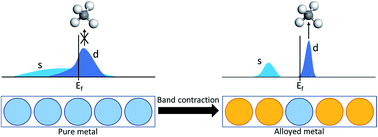
The catalytic conversion of methane under mild conditions is an appealing approach to selectively produce value-added products from natural gas. Catalysts which can chemisorb methane can potentially overcome challenges associated with its high stability and achieve facile activation. Although transition metals can activate C–H bonds, chemisorption and low-temperature conversion remain elusive on these surfaces. The broad electronic bands of metals can only weakly interact with the methane orbitals, in contrast to specific transition metal oxide and supported metal cluster surfaces which are now recognized to form methane σ-complexes. Here, we report methane chemisorption can, remarkably, occur on metal surfaces via electronic band contraction and localization from metal alloying. From a broad screening including single atom and intermetallic alloys in various substrates, we find early transition metals as promising metal solutes for methane chemisorption and low-temperature activation. These findings demonstrate a combinatorial diversity of possible candidates in earth abundant metal alloys with this attractive catalytic behavior.


 Please wait while we load your content...
Please wait while we load your content...