Organic polymeric filler-amorphized poly(ethylene oxide) electrolyte enables all-solid-state lithium–metal batteries operating at 35 °C†
Abstract
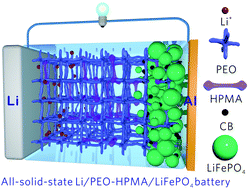
The poor ionic conductivity and high working temperatures (normally >60 °C) of poly (ethylene oxide) (PEO)-based solid polymer electrolytes (SPEs) greatly limit their application in all-solid-state batteries. To mitigate these issues, for the first time, we report here an organic polymer filler, hydrolyzed polymaleic anhydride (HPMA), that can greatly suppress PEO crystallinity, enhance the ionic conductivity of PEO-based SPEs (1.13 × 10−4 S cm−1 at 35 °C) and support battery operation at 35 °C. PEO–HPMA SPEs feature high flexibility, incombustibility, wide electrochemical operating window and stability against lithium. The as-derived Li/PEO–HPMA/LiFePO4 all-solid-state batteries show outstanding rate capability, high reversible capacity and long-term stability up to 1250 cycles. More impressively, the soft-packaged Li/PEO–HPMA/LiFePO4 cells show high safety under various extreme conditions such as cutting and perforation. The PEO–HPMA SPE-based quasi-solid-state lithium-sulfur batteries are also presented. This work demonstrates a facile approach that unlocks the low-temperature application of PEO SPE-based all-solid-state batteries.


 Please wait while we load your content...
Please wait while we load your content...