Boron enhances oxygen evolution reaction activity over Ni foam-supported iron boride nanowires†
Abstract
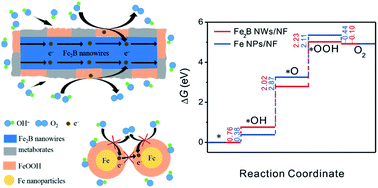
Transition metal borides are one of the most promising electrocatalysts for oxygen evolution reaction (OER), but the precise role of boron is still not understood. Herein, we demonstrate experimentally and computationally that boron facilitates OER by modulating the interaction energies of the reaction intermediates. Through a simple chemical reduction in an externally applied magnetic field, one-dimensional (1D) Fe2B nanowires (NWs) can be directly deposited onto a three-dimensional (3D) nickel foam (NF). OER in alkaline solution converts interconnected Fe2B NWs to a thicker and larger network of metaborate- and FeOOH-covered Fe2B NWs. The Fe2B NWs/NF-based catalyst lowers the overpotential to 276 mV at 10 mA cm−2 (normalized to the electrochemical surface area). This intrinsic catalytic activity ranks top among the transition-metal-based compounds. This catalyst is highly stable, showing only 6 mV change in overpotential over continuous testing for 11 h. Such high efficiency is mainly attributed to the catalyst's unique electronic structure, accelerated charge transport, and hydrophilic surface. The remarkable stability stems from the increase in corrosion resistance by the metaborate species in the catalyst.


 Please wait while we load your content...
Please wait while we load your content...