Isomeric effect of fluorene-based fused-ring electron acceptors to achieve high-efficiency organic solar cells†
Abstract
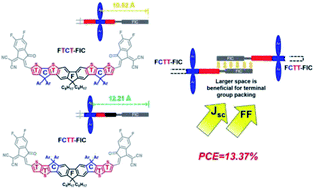
Acceptor–donor–acceptor (A–D–A) non-fullerene electron acceptors (NFEAs) using ladder-type donor structures have become the dominant n-type materials for achieving high-efficiency OSCs. In this work, two isomeric fluorene-based ladder-type structures FCTT (TT-C-F-C-TT) and FTCT (T-C-TFT-C-T) have been designed and synthesized. These two isomeric donors with the different fused-ring arrangement, molecular geometry, and side-chain placement were end-capped with the FIC acceptors to form two NFEAs FCTT-FIC and FTCT-FIC isomeric materials. Compared to FTCT-FIC using the thiophene (T)-terminal donor, FCTT-FIC with the thienothiophene (TT)-terminal donor has more evenly distributed side chains on both sides of the backbone and less steric hindrance near the FIC acceptors, which enables stronger antiparallel π–π packing among the end-groups to create a channel for efficient electron transport, as evidenced by the thin-film GIWAXS measurements. FCTT-FIC displayed a larger optical bandgap and deeper-lying energy levels than its FTCT-FIC isomer. Compared to the PBDB-T:FTCT-FIC device, the PBDB-T:FCTT-FIC device showed a higher PCE of 10.32% with an enhanced Jsc of 19.63 mA cm−2 and an FF of 69.14%. A PM6:FCTT-FIC device using PM6 as a p-type polymer achieved the highest PCE of 12.23%. By introducing PC71BM as the second acceptor to enhance the absorption at shorter wavelengths, optimize the morphology and facilitate electron transport, the ternary-blend PM6:FCTT-FIC:PC71BM (1 : 1 : 0.5 in wt%) device yielded the highest PCE of 13.37% with a Voc of 0.92 V, a higher Jsc of 19.86 mA cm−2, and an FF of 73.2%. This result demonstrated that the TT-terminal ladder-type donor is generally a better molecular design than the corresponding T-terminal ladder-type isomer for the development of new A–D–A NFEAs.


 Please wait while we load your content...
Please wait while we load your content...