Two-step pyrolytic engineering of carbon-doped Co3O4 with rich defects for efficient low-temperature CO oxidation†
Abstract
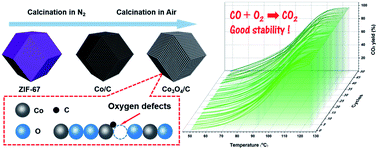
A series of carbon-doped Co3O4 samples with rich defects are rationally synthesized by two-step pyrolysis of ZIF-67 in different atmospheres. Specifically, Co3O4/C-300 exhibits the lowest apparent activation energy (Ea = 47.6 kJ mol−1) and the highest activity (T100% = 120 °C) for CO oxidation, as well as excellent catalytic stability for 50 cycles. The intensive investigation of the structural and physicochemical properties of the catalysts demonstrates that the excellent catalytic performance is predominantly attributed to the synergistic effects of abundant active Co3+ sites, good low-temperature reducibility, more active oxygen species and better oxygen storage capacity (OSC). Besides, the in situ DRIFTS analysis reveals that CO is oxidized by Oads on the surface active sites of the material to finally form CO2. Thus, these findings may shed light on the design of carbon-doped metal oxides to approach reactions with superior performance by tuning the defect concentration of the material.


 Please wait while we load your content...
Please wait while we load your content...