Dynamic evolution of a hydroxylated layer in ruthenium phosphide electrocatalysts for an alkaline hydrogen evolution reaction†
Abstract
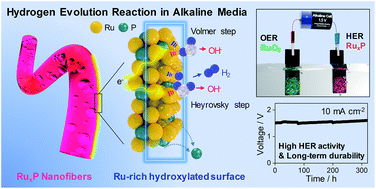
Alkaline water electrolysis represents one of the most promising technologies for the development of environment-friendly energy cycles. Ruthenium phosphide electrocatalysts are attractive candidates for this process, and they recently showed high electrocatalytic activity for the hydrogen evolution reaction (HER) in alkaline conditions, which is even higher than that in acidic conditions; however, the origin of their activity has not been addressed to date. Here, we demonstrate that hydroxylated Ru species reconstructed by HER in a basic electrolyte are the key active sites for alkaline HER based on an in-depth X-ray photoelectron spectroscopic study. Ru phosphides with a higher Ru/P ratio in their bulk composition possess a higher ratio of hydroxylated Ru on their surface region of several nanometers with less P sites exposed, which determines the HER activity in alkaline conditions. The Ru phosphide nanofiber electrocatalysts presented here enabled almost zero overpotentials for alkaline HER with stable performance for 320 h. This work provides a deeper understanding of the origin of high HER activity in alkaline conditions.


 Please wait while we load your content...
Please wait while we load your content...