Tailorable nanoarchitecturing of bimetallic nickel–cobalt hydrogen phosphate via the self-weaving of nanotubes for efficient oxygen evolution†
Abstract
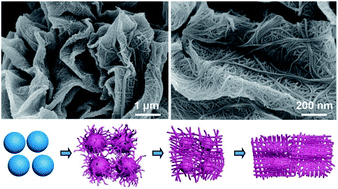
This study demonstrates the tailorable self-weaving of bimetallic nickel–cobalt (Ni–Co) hydrogen phosphate nanotubes into one-dimensional (1D) microspindles or two-dimensional (2D) sheet-like structures by utilizing monodispersed Ni–Co glycerate spheres as sacrificial templates. The conversion process is achieved through a two-step solvothermal method in the presence of phosphoric acid (H3PO4) as a phosphorus source and promoter of the self-weaving process. The formation of such nanotube-assembled architectures is promoted by the “peeling-self-weaving” mechanism, in which the bimetallic Ni–Co hydrogen phosphate nanotubes initially grow on the surface of the Ni–Co glycerate spheres due to the reactions between Ni and Co metals bonded to the glycerate anions with hydrogen phosphate anions present in the solution. This is followed by the peeling of the overgrown nanotubes from the etched glycerate spheres and their self-weaving into 1D or 2D architectures depending on the Ni/Co molar ratio. The electrocatalytic test results reveal the superior activity of the Ni-rich Ni–Co hydrogen phosphate electrode for oxygen evolution reaction (OER) compared to its Co-rich and equimolar counterparts, leading to smaller overpotential of 320 mV and lower Tafel slope of 84 mV dec−1. Post-OER analysis of this sample reveals that the high OER activity is derived from the formation of active Ni–Co oxyhydroxide phase on its surface.


 Please wait while we load your content...
Please wait while we load your content...