Increased activity in the oxygen evolution reaction by Fe4+-induced hole states in perovskite La1−xSrxFeO3†
Abstract
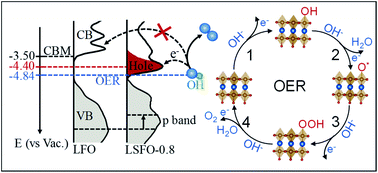
Perovskite transition metal oxides are promising non-precious metal electrocatalysts for promoting the oxygen evolution reaction (OER) in many electrochemical energy conversion devices. This work reports a systematic study of the relation between the electronic structure and OER performance of perovskite La1−xSrxFeO3 (0 ≤ x ≤ 1). The partial substitution of La for Sr in LaFeO3 results in the oxidation of Fe3+ to Fe4+ and substantially enhances OER activity. A comprehensive X-ray spectroscopic study reveals a strong correlation of the enhanced OER activity with these Fe4+ states. The presence of Fe4+ leads to a stronger Fe–O bond due to stronger Fe 3d–O 2p orbital hybridization and shifts the energy position of the valence band (VB) to EF. Such an electronic modulation optimizes the surface adsorption energetics of *OH intermediates, contributing to faster OER kinetics. Furthermore, a new unoccupied state is created at ∼0.9 eV below the conduction band. This hole state reduces the energy barrier for electron transfer from 1.34 eV to 0.44 eV, thereby facilitating charge transfer at the interface. The Fe4+-induced electronic states responsible for higher OER activity have implications for the understanding of structure–activity relationships in other Fe-based electrocatalysts such as highly active Fe-doped NiOOH.


 Please wait while we load your content...
Please wait while we load your content...