Strain effects on the Co oxidation state and the oxygen dissociation activity in barium lanthanum cobaltite thin films on Y2O3 stabilized ZrO2†
Abstract
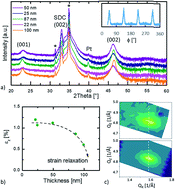
Cobaltite oxides, like Ba(La)CoO3, are very promising candidates as cathode materials for solid oxide fuel cells and electrolysers (SOFCs and SOECs). Lattice distortions have been shown as a promising approach to improve cathodic properties. To investigate this possible approach, biaxial strain is induced on model systems, i.e. through the epitaxial growth of barium lanthanum cobaltite on single crystalline substrates. The influence of strain on the oxidation state of cobalt is probed through energy electron loss spectroscopy. XRD measurements indicate that films with biaxial compressive strain exhibit a larger unit cell volume. Electron energy-loss spectroscopy (EELS) shows that biaxial compressive strain induces a higher Co2+/Co3+ ratio, probably enabled through the larger ionic radius of Co2+. Electrical conductivity relaxation measurements reveal that the activation energy for the surface exchange is decreased with the increasing amount of Co2+. This strongly indicates the connection between the unit cell volume, the oxidation state of cobalt, and the surface exchange kinetics of Ba(La)CoO3. An improved understanding of the fundamental kinetics in cathode materials for SOFCs is important for further materials development.


 Please wait while we load your content...
Please wait while we load your content...