Photocatalytic hydrogen evolution on a high-entropy oxide
Abstract
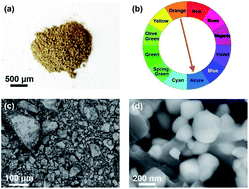
The introduction of high-entropy oxides (HEOs), i.e. compounds containing oxygen and five or more cations in their crystal structure, has led to interesting functional properties in recent years. In this study, the first high-entropy photocatalyst is synthesized by mechanical alloying via the high-pressure torsion (HPT) method followed by high-temperature oxidation. The synthesized oxide contains 60 mol% of AB2O7 monoclinic perovskite and 40 mol% of A6B2O17 orthorhombic perovskite, where A represents Ti, Zr and Hf and B represents Nb and Ta. This two-phase oxide with an overall composition of TiHfZrNbTaO11 and a d0 electronic configuration shows an appreciable light absorbance in the visible-light region with a bandgap of 2.9 eV and appropriate valence and conduction bands for water splitting. The material successfully produces hydrogen by photocatalytic water splitting, suggesting the potential of HEOs as new low-bandgap photocatalysts.
- This article is part of the themed collection: Energy Frontiers: Hydrogen


 Please wait while we load your content...
Please wait while we load your content...