High-performance 3 V “water in salt” aqueous asymmetric supercapacitors based on VN nanowire electrodes†
Abstract
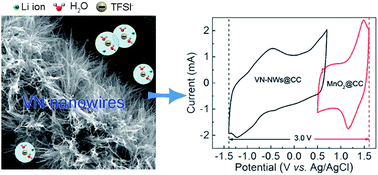
Aqueous supercapacitors had recently received widespread attention due to their eco-safety, cost-efficiency, environmental friendliness, and ease of handling. However, being limited by the voltage of decomposition of water, the operating voltage of aqueous supercapacitors is usually 2 V, which mostly impedes the improvement of energy density (E = 0.5 CV2). In the present study, we illustrate that a VN nanowires@carbon cloth (VN-NWs@CC) electrode possesses a wide potential stability window of −1.4–0.7 V in a high concentration “water in salt” aqueous electrolyte, which can be attributed to the redox reactions occurring at low potentials, delaying the process of hydrogen evolution reaction (HER). Furthermore, the asymmetric supercapacitor, based on a VN-NWs@CC negative electrode and MnO2 nanosheet positive electrode, can obtain a high operating voltage of 3 V. Moreover, the 3 V MnO2@CC//VN-NWs@CC asymmetric supercapacitor exhibits a large energy density of up to 61.5 W h kg−1 with excellent cycling stability, outperforming previously reported VN-based supercapacitors. This work provides the possible chance for providing high operating voltage for aqueous supercapacitors, comparable even with those of batteries.


 Please wait while we load your content...
Please wait while we load your content...