Electrochemical formation of PtRu bimetallic nanoparticles for highly efficient and pH-universal hydrogen evolution reaction†
Abstract
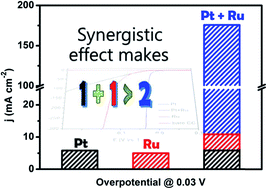
The production of hydrogen by electrolyzing water, which stores the excess temporal fluctuations of solar and wind power in the form of chemical energy, is usually energy intensive and expensive. Therefore, developing highly efficient and low-cost electrocatalysts via a simple synthesis strategy for the hydrogen evolution reaction (HER) has become a remarkable challenge in energy and environmental science. Herein, we report a new simple electrochemical deposition (ECD) method for the synthesis of a highly efficient carbon-cloth-supported PtRu HER catalyst (PtRu/CC1500) in a wide pH range, in which Pt in an ultralow loading amount (1.6 μg cmCC−2) is electrodeposited on Ru nanoparticles (NPs). This catalyst presents remarkable performance, with low overpotentials of only 8, 25 and 19 mV at 10 mA cm−2 (η10) in 0.5 M H2SO4, 1 M phosphate buffer solution (PBS) and 1.0 M KOH, respectively, as well as long-term stability (10 000 cycles). Also, even at a high current density of 300 mA cm−2, it still displays small overpotentials of 37 and 95 mV in 0.5 M H2SO4 and 1.0 M KOH solution, respectively. In addition, the PtRu catalyst achieves an excellent mass current density of 109.9 mA μg−1 (in 0.5 M H2SO4 at −30 mV) and 198.7 mA μg−1 (in 1.0 M KOH at −100 mV), which are 30 and 59 times higher than those of a commercial Pt/C catalyst, respectively. Density functional theory calculations suggest that PtRu nanoparticles (NPs) not only significantly improve hydrogen desorption (ΔGH* very close to zero) but also promote H2O dissociation, accounting for their enhanced catalytic activity. Due to the simple preparation strategy, excellent HER performance and long-term durability of the PtRu catalyst, it has great potential for commercial applications.


 Please wait while we load your content...
Please wait while we load your content...